
Fusen pharmaceutical: metformin hydrochloride sustained release tablets passed the consistency evaluation
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-04-26
- Views:
(Summary description) Fusen pharmaceutical metformin hydrochloride sustained-release tablets have successfully passed the evaluation of the consistency of quality and efficacy of generic drugs! Metformin Hydrochloride Sustained Release Tablets, a diabetes drug, has been approved by the State Administration for drug control. This product has successfully passed the quality and efficacy consistency evaluation of generic drugs, which is an authoritative recognition of the R & D capacity, production and quality management system and clinical efficacy of Fusen pharmaceutical, further highlights the product brand of Fusen pharmaceutical, helps to improve the market competitiveness of the drug, and accumulates valuable experience for the company's follow-up products to carry out generic drug consistency evaluation. ▲ notice of approval from the State Drug Administration Metformin Hydrochloride Tablets is the basic medicine for diabetes. It is used for diet and physical exercise to control type 2 diabetes patients with blood glucose inefficacy, especially obesity and hyperinsulinemia. As a first-line drug for the treatment of type II diabetes, metformin is often used in combination with other oral hypoglycemic agents. ▲ for medical professionals only Because of the superiority of metformin, it is listed as the first-line treatment for hypoglycemic treatment in the guidelines for prevention and treatment of type II diabetes, formulated by professional academic organizations both at home and abroad. As the holder of drug marketing license, Fusen pharmaceutical will not forget its original intention, forge ahead, strictly control the product quality, and constantly optimize the product layout, so that more drugs of Fusen pharmaceutical can benefit the majority of patients.
Fusen pharmaceutical: metformin hydrochloride sustained release tablets passed the consistency evaluation
(Summary description)
Fusen pharmaceutical metformin hydrochloride sustained-release tablets have successfully passed the evaluation of the consistency of quality and efficacy of generic drugs!
Metformin Hydrochloride Sustained Release Tablets, a diabetes drug, has been approved by the State Administration for drug control. This product has successfully passed the quality and efficacy consistency evaluation of generic drugs, which is an authoritative recognition of the R & D capacity, production and quality management system and clinical efficacy of Fusen pharmaceutical, further highlights the product brand of Fusen pharmaceutical, helps to improve the market competitiveness of the drug, and accumulates valuable experience for the company's follow-up products to carry out generic drug consistency evaluation.
▲ notice of approval from the State Drug Administration
Metformin Hydrochloride Tablets is the basic medicine for diabetes. It is used for diet and physical exercise to control type 2 diabetes patients with blood glucose inefficacy, especially obesity and hyperinsulinemia. As a first-line drug for the treatment of type II diabetes, metformin is often used in combination with other oral hypoglycemic agents.
▲ for medical professionals only
Because of the superiority of metformin, it is listed as the first-line treatment for hypoglycemic treatment in the guidelines for prevention and treatment of type II diabetes, formulated by professional academic organizations both at home and abroad.
As the holder of drug marketing license, Fusen pharmaceutical will not forget its original intention, forge ahead, strictly control the product quality, and constantly optimize the product layout, so that more drugs of Fusen pharmaceutical can benefit the majority of patients.
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-04-26 10:42
- Views:

Fusen pharmaceutical metformin hydrochloride sustained-release tablets have successfully passed the evaluation of the consistency of quality and efficacy of generic drugs!
Metformin Hydrochloride Sustained Release Tablets, a diabetes drug, has been approved by the State Administration for drug control. This product has successfully passed the quality and efficacy consistency evaluation of generic drugs, which is an authoritative recognition of the R & D capacity, production and quality management system and clinical efficacy of Fusen pharmaceutical, further highlights the product brand of Fusen pharmaceutical, helps to improve the market competitiveness of the drug, and accumulates valuable experience for the company's follow-up products to carry out generic drug consistency evaluation.
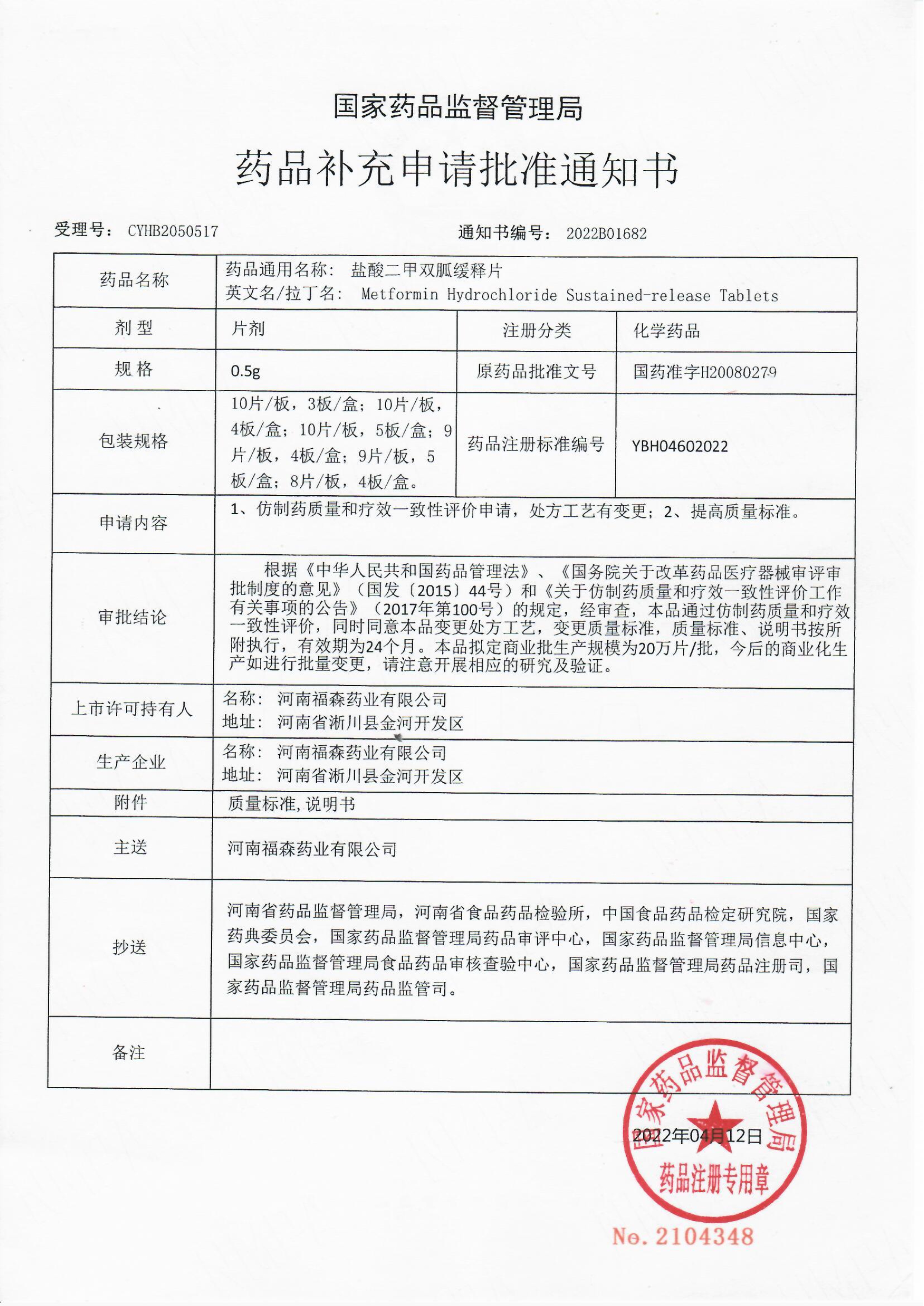
▲ notice of approval from the State Drug Administration
Metformin Hydrochloride Tablets is the basic medicine for diabetes. It is used for diet and physical exercise to control type 2 diabetes patients with blood glucose inefficacy, especially obesity and hyperinsulinemia. As a first-line drug for the treatment of type II diabetes, metformin is often used in combination with other oral hypoglycemic agents.
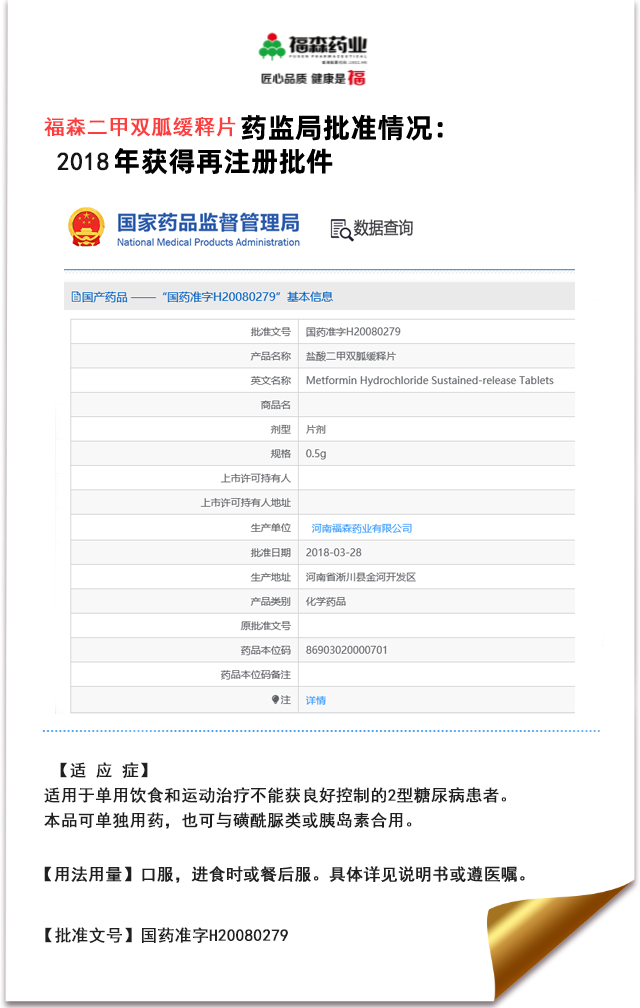
▲ for medical professionals only
Because of the superiority of metformin, it is listed as the first-line treatment for hypoglycemic treatment in the guidelines for prevention and treatment of type II diabetes, formulated by professional academic organizations both at home and abroad.
As the holder of drug marketing license, Fusen pharmaceutical will not forget its original intention, forge ahead, strictly control the product quality, and constantly optimize the product layout, so that more drugs of Fusen pharmaceutical can benefit the majority of patients.
Related News

Copyright © 2021 Fusen Pharmaceutical Company Limited
Copyright © 2021 Fusen Pharmaceutical All Rights Reserved. 豫ICP备2021028871号 Powered by www.300.cn




 WhatsApp
WhatsApp Skype
Skype +86-377-62002535
+86-377-62002535 fusenyaoye2003@126.com
fusenyaoye2003@126.com

 Messages
Messages 